PBI acquired the TriGrid Delivery System
Papivax Biotech, Inc. (PBI) announced today that it has completed acquisition of the TriGrid® Delivery System (TriGrid) from Ichor Medical Systems, including the core technology for electroporation mediated delivery of DNA-based biologics, non-clinical and clinical devices, and all of the associated intellectual property. The TriGrid technology is distinguished as an integrated, automated administration device that is simple to use and can dramatically enhance the potency of DNA based vaccines and immunotherapeutics. The device technology been utilized as the means of DNA administration in more than 30 clinical studies, including multiple randomized comparative studies demonstrating enhanced magnitude, breadth and frequency of immune response following DNA vaccination with the TriGrid versus conventional injection.
PBI is advancing the development of multiple immunotherapeutic candidates for the treatment of patients with diseases related to chronic infection with human papilloma virus (HPV). Persistent HPV infections are associated with high risk of various diseases, including cervical cancer, other genital cancers, oropharyngeal cancers, genital warts, and recurrent respiratory papillomatosis. While current vaccines are capable of preventing HPV infection, they are ineffective in clearing the virus in women who are already infected with HPV. Despite advances in screening and preventative HPV vaccination, a significant number of women still develop advanced disease.
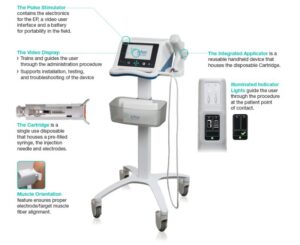
Under a prior research agreement, PBI evaluated the TriGrid technology as a means to deliver its DNA-based HPV vaccine candidates and molecular adjuvants. Based on the favorable outcome of these initial studies, PBI elected to acquire the TriGrid technology and associated intellectual property. As part of the acquisition, PBI has established a wholly owned U.S. subsidiary (PBI US, Inc.) that will be responsible for the continued development and deployment of the technology. Key objectives for PBI following the acquisition will include expanded clinical evaluation of the TriGrid technology for delivery of its HPV immunotherapy candidates as well as ongoing support for all ongoing TriGrid partnerships and licenses using the technology outside of PBI’s core HPV related disease indications.
PBI CEO Lily Wu stated, “We are extremely excited about the data that has been generated with the TriGrid delivery of our candidates. TriGrid delivery of HPV antigens and our potent molecular adjuvants appears capable of inducing the T-cell responses necessary to clear persistent HPV infections. By ensuring that we have full ownership and control of all of the core elements of our HPV immunotherapy regimens, this acquisition greatly facilitates our path to commercialization. PBI also looks forward to leveraging our core immunotherapy technology platforms to assist existing and prospective partners address other unmet medical needs in oncology and chronic infection.”