To support the development of its immunotherapy candidates, PBI has assembled a portfolio of technologies that enable induction of robust antigen-specific cellular immune responses in the clinical setting. The PBI immunotherapy platform includes four key elements:
(1) DNA Vaccines Encoding Potent Molecular Adjuvants:
DNA vaccines are a favorable platform for therapeutic immunization because they are capable of inducing antigen-specific T cell responses, they are simple to manufacture and very stable compared to other biological agents, do not require complex formulations, have an excellent safety profile, and can be repeatedly administered without the induction of inhibitory anti-vector responses. Combined with the inherent ability to induce sustained expression of the target antigen with just a single dose administration, DNA vaccines are well suited to induce and sustain target immunological responses. DNA vaccines also facilitate the incorporation of adjuvants into a vaccine design without complex manufacturing or formulation issues. An adjuvant is a substance that is administered in the context of a vaccine or immunotherapy to enhance a recipient’s response to the target antigens. Researchers at JHU performed extensive screening of a variety of materials in order to identify candidate adjuvants capable of enhancing the induction of immune responses against HPV antigens and other targets. The most promising adjuvants identified were substances known as heat shock proteins (HSPs) (either HSP70 from a bacterium (M.bovis) or the human calreticulin, a type of HSP) which were able to increase immune response by over 50 times compared to the antigen alone.
Mechanistic analysis suggest that fusion of the HSP adjuvants to the target antigen(s) of interest can profoundly enhance the fused antigen-specific immune response through multiple pathways (Figure 1). Specifically, the HSP renders fused HPV antigens highly immunogenic by promoting uptake by antigen presenting cells where they can be processed and presented to the immune effector cells which can then promote clearance of infected cells. In addition to promoting, antigen processing and presentation the HSP adjuvant also acts as a ‘red flag’ to the immune system and promote upregulation of immunostimulatory signals.
Use of the HSPs as adjuvants is covered by patents that were originally licensed from JHU and the vaccines have been safely used in several clinical studies with promising results. PBI has been awarded its own follow-on patents for improvements to the technology and methods of use.
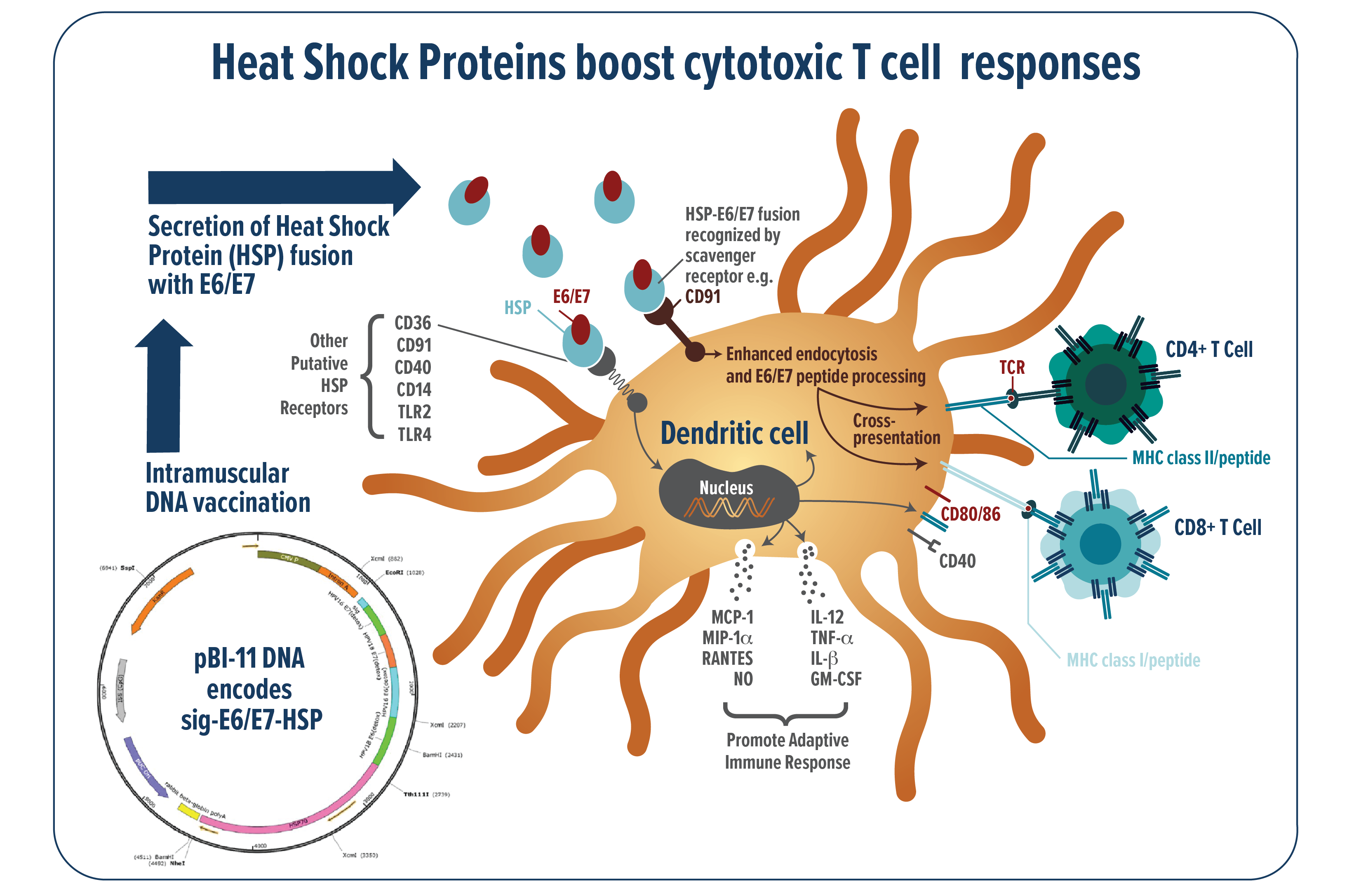
FIGURE 1: Schematic of the patented Heat Shock Protein technology
(2) Robust Vector / Delivery Technology:
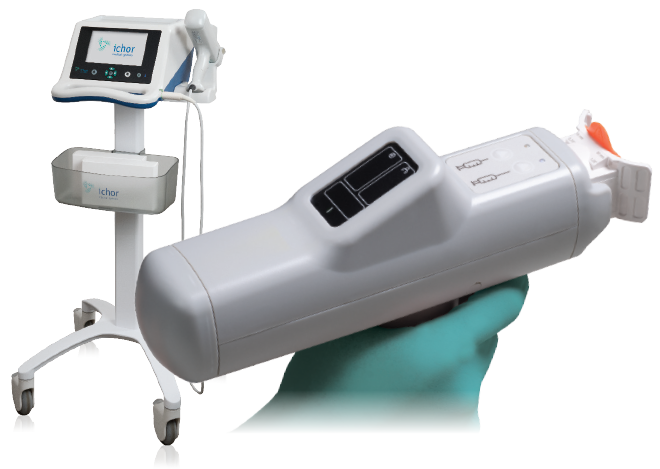
The TriGrid® Delivery System is a technologically superior, clinically tested, is an electroporation based technology that can dramatically enhance the potency of DNA-based biologics. By integrating the means for agent administration and electroporation application into a single, automated device, the TriGrid® technology enables consistent delivery in a manner capable of supporting commercialization of the biologics it is used to deliver.
Originally developed by Ichor Medical Systems, PBI acquired the TriGrid technology for the delivery of its DNA based vaccine candidates for the treatment of HPV related diseases. The TriGrid® technology has been licensed by academics and commercial developers to deliver their proprietary DNA vaccines. The technology has also been extensively evaluated for DNA-based vaccine antibody delivery. PBI continues to seek strategic partnerships to provide the TriGrid® technology to partners interested in expanding their product pipeline in DNA-based biologics.
The TriGrid® Delivery System is an investigational device designed to enhance the intracellular delivery of nucleic acid constructs in skeletal muscle. The system is comprised of three main components as shown in Figure 2:
- The Pulse Stimulator controls the administration sequences and generates the electroporation pulses.
- The Integrated Applicator is a reusable handheld device that houses the single-use cartridge. It automatically deploys the electrodes and initiates administration of the biologic agent at the touch of a single button. Its automated design ensures a reproducible application procedure with minimal operator training.
- The Application Cartridge is a sterile, single-use component that houses the nucleic acid construct to be administered and a TriGrid® electrode array for the electroporation procedure.
FIGURE 2: TriGrid Delivery System
APPLICATIONS
The TriGrid® technology provides a delivery solution for nucleic acid candidates in two broad categories:
1. Nucleic Acid Vaccines / Immunotherapeutics
- Alternative to traditional vaccine approaches
- Prevention of Infectious Diseases
- Immunotherapy / novel vaccine indications
- Oncology
- Chronic viral infection
- Autoimmune disease, allergy
2. Protein / Monoclonal antibody (mAb) delivery
- Alternative to systemic delivery of recombinants
- Approved and/or novel agents
- Low cost, sustained passive immunity (mAb)
- Locoregional protein expression
- Growth factors for revascularization / wound healing
- Immunomodulators
(3) Recombinant vaccinia vector:
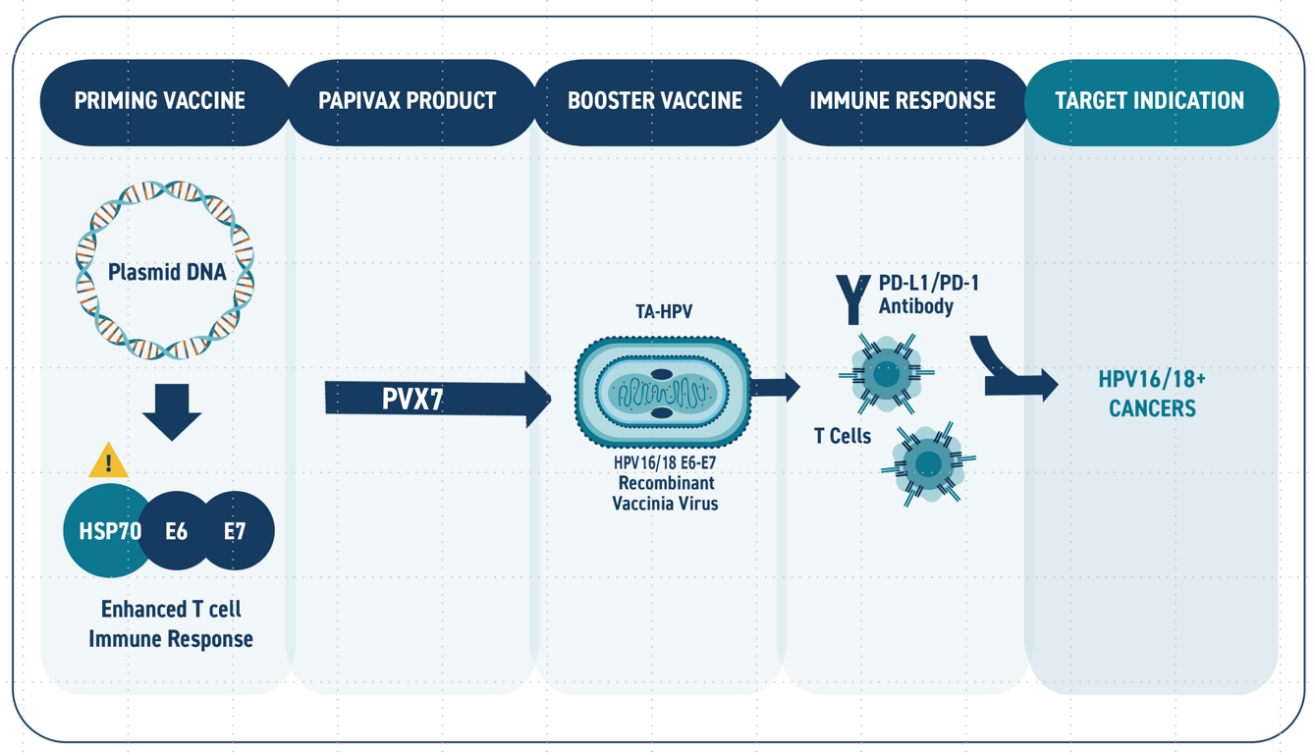
While the PBI DNA vaccine candiates for HPV-related diseases have demonstrated the ability to induce potent T cell responses and viral clearance in human clinical studies, further enhancement in the immunotherapy regimen may be necessary to maximize response rate in patients with late stage disease (i.e., cancer) and/or compromised immune function. Several clinical studies have demonstrated that an immunization regimen comprising a combination of a DNA vaccine and a viral vector can provide quantitative and qualitative improvements in immune response compared to the administration of either modality alone. In order to provide an additional platform to support development of its HPV immunotherapy franchise, PBI licensed from Cancer Research UK a recombinant attenuated vaccinia viral vector (TA-HPV) as a “boost” immunization to further enhance the immune responses induced by its DNA vaccine candidates as shown in Figure 3. TA-HPV is replication-competent and has a well understood safety profile based on use in phase II studies. Notably, the backbone vector has been used in global immunization campaigns. PBI has obtained method patents (US10512683) covering use of the combined regimens which could be utilized in disease indications and/or patient subpopulations where a single modality is not sufficient to achieve target levels of disease response.
FIGURE 3: Schematic of the PVX7 prime-boost immunotherapy regimen
(4) Systemic Immunomodulators:
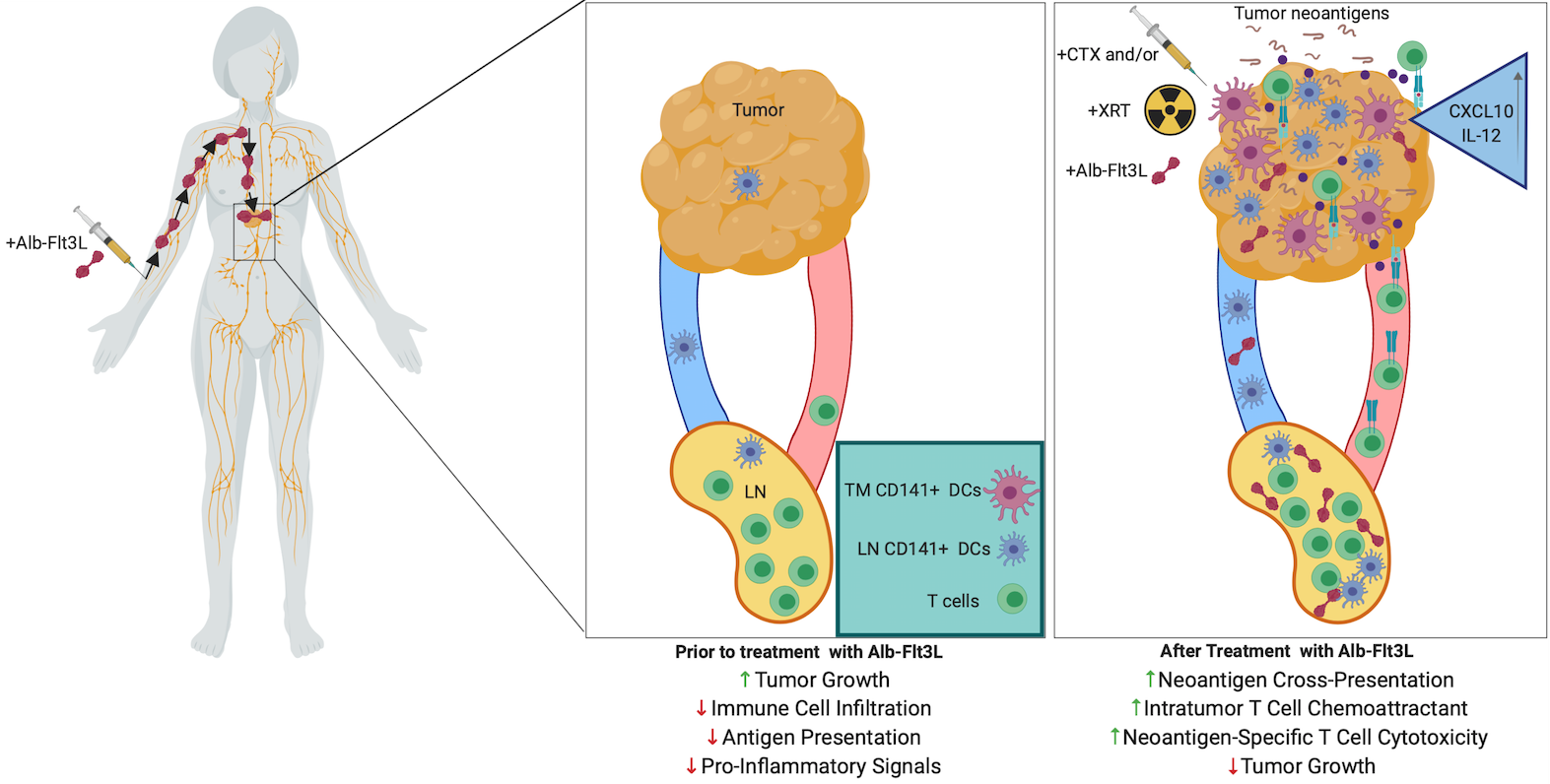
More recently. PBI has licensed a technology from Johns Hopkins University, which is capable of recruiting and expanding professional antigen-presenting cells including dendritic cells (DCs) to the location of tumors and draining lymph nodes. In cancer, DCs are indispensable for T cell activation, so consequently there is a restriction on cytotoxic T cell immunity if DCs are not present in sufficient numbers in the tumor and draining lymph nodes to take up and present relevant cancer antigens. To address this bottleneck, PBI has licensed a technology developed at Johns Hopkins University. The technology is based on albumin fused with FMS-related tyrosine kinase 3 ligand (Alb-Flt3L) that demonstrated superior pharmacokinetic properties compared with Flt3L, including significantly longer half-life, accumulation in tumors and lymph nodes, and cross-presenting-DC expansion following a single injection. It has been shown that Alb-Flt3L, in combination with standard-of-care chemotherapy and radiation therapy, serves as an in situ vaccination strategy capable of engendering polyclonal tumor neoantigen–specific immunity spontaneously. In addition, Alb-Flt3L–mediated tumor control synergized with immune checkpoint blockade delivered as anti–PD-L1. The Alb-Flt3L can also be delivered through DNA expressing vector with electroporation to generate comparable therapeutic antitumor effects in preclinical model. Taken together, the acquisition of Alb-Flt3L techmology by PBI can be used with PBI’s TriGrid platform technology to create a new platform technology for the development of immunotherapies against cancers.
FIGURE 4: Schematic of the Alb-Flt3L immunotherapeutic mechanism