PBI’s therapeutic vaccines are designed to activate potent anti-viral immune responses to effectively kill HPV-infected precancerous (dysplastic) or cancerous cells and cure the patient. This approach is safe because it does not target uninfected normal cells, and effective because the virus is both readily recognized as foreign by the immune system and required for the growth of the cancer cells so they cannot readily evade anti-viral immune responses.
Women are typically screened annually with a Pap smear (a.k.a. Pap test) for the presence of cervical precancerous and cancerous cells. For certain diagnoses this is supplemented with one of several FDA-approved HPV tests to determine whether the type of HPV present is high risk, and in particular to detect HPV16. Our lead program uses the PVX2 immunotherapy regimen to treat patients with known chronic HPV16 cervical infection associated with low-grade precancerous lesions (termed ASC-US/ASC-H/LSIL/CIN1 by pathologists). Currently, there is no effective treatment to offer these asymptomatic patients with persistent HPV16 cervical infection and early precancerous lesions. Instead they are followed with “watchful waiting” by repeat testing every 6~12 months for high grade precancer or cancer. This causes patients considerable anticipatory anxiety and stigma-related stress and shame ref. Since in only 9% of these cases the host immune system will spontaneously clear the HPV16 infection by 6 months ref, these patients clearly have an unmet need for (and want) a medical treatment for this sexually-transmitted and cancer-causing infection. PBI is treating these patients with PVX2 to trigger HPV16 clearance and thereby eliminate its potential to drive progression to high-grade precancer (CIN2-3) and/or invasive and metastatic cancer.
Normal cervical mucosa infected by hrHPV with subsequent evolution to low-grade precancer, high-grade precancer, and eventually to invasive cancer that metastasizes
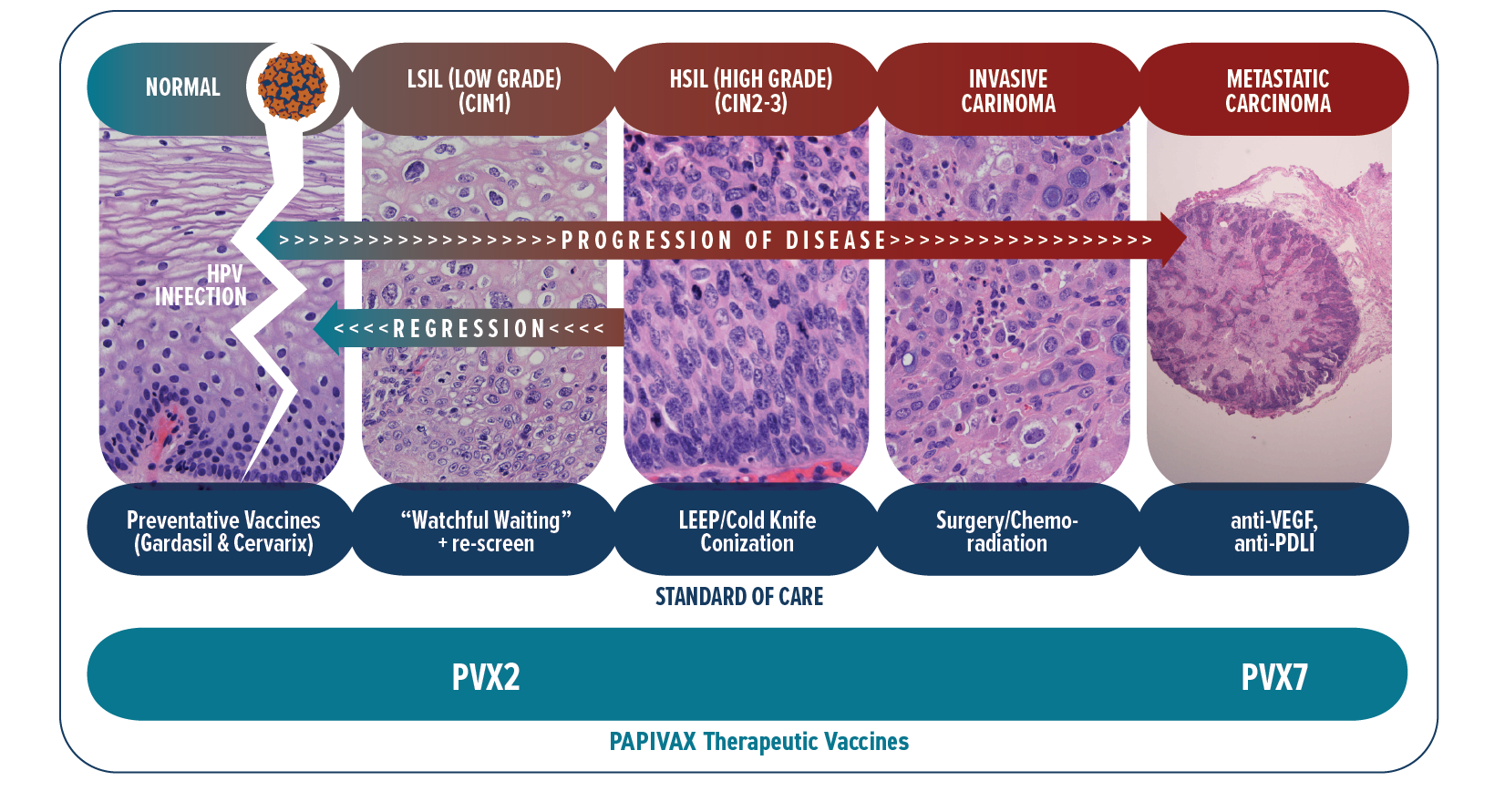
Patients with high-grade precancer (CIN2-3) must undergo a surgery (loop electrosurgical excision procedure [LEEP] or cold-knife conization) or ablation (laser or cryotherapy) in which the mouth of the womb (the uterine cervix) is removed. This surgery is effective generally, although the disease does re-occur in a significant fraction of cases and infection can spread to other genital sites (vulva, vagina and anus) susceptible to cancer. Importantly, the surgery is associated with complications of an incompetent cervix during pregnancy (requiring suturing up of the cervix) and premature delivery of babies which can be harmful for them. Additional complications include infections at the surgical site, intrauterine fetal infections and abortion. PVX2 is intended to eliminate the need for the surgery, and academic studies are also exploring the use of our therapeutic HPV vaccination as a safer alternative to replace this surgery.
Patients with invasive cervical cancer are treated with surgical resection, radiotherapy and/or chemotherapy depending on how far the cancer has spread. Unfortunately, a subset of patients will not be cured. When these treatments fail, patients can now receive a new immune checkpoint therapy targeting the PD-1/PD-L1 pathway. While these immune checkpoint inhibitors have in recent years improved disease control and survival in these patients with advanced cervical cancer, such response has been observed only in a small and insufficient subset (12~26%) of patients ref. This treatment works by unleashing a pre-existing spontaneous anti-HPV/tumor immune response. Unfortunately, most patients do not have a robust pre-existing spontaneous anti-HPV/tumor immune response and thus this immune checkpoint therapy treatment fails these patients. PBI has developed PVX7 for vaccination against HPV16/18 to trigger the needed anti-HPV/tumor immune response that can be further enhanced by the immune checkpoint therapy. This approach will also be tested for HPV-associated advanced head & neck cancer, of which 90% is caused by HPV16 ref.