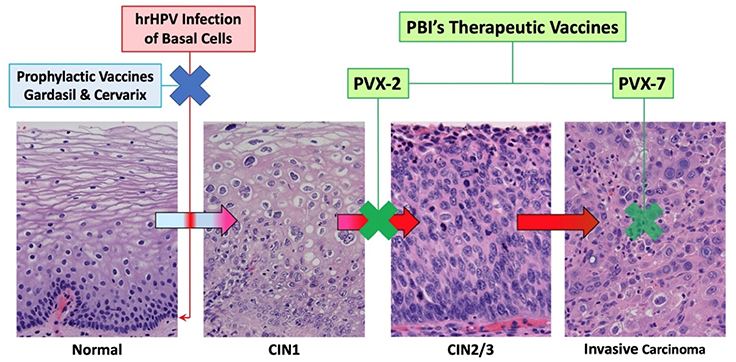
The US FDA approved several preventive (a.k.a. prophylactic) HPV vaccines, firstly Gardasil© (by Merck; against HPV6, 11, 16 and 18) and Cervarix© (by GlaxoSmithKline or GSK; against HPV16 and 18) in 2006 and 2009, respectively, for use in adolescents for prevention of hrHPV-associated cervical cancer. Gardasil© was updated to Gardasil 9© and approved in 2014 for use in young adults up to age 26. In 2018, its use was extended to adults up to age 45 for prevention of HPV-associated cervical, vulvar, vaginal, anal, and oropharyngeal/head and neck cancers, but the focus remains on vaccinating adolescents prior to sexual debut. These vaccines have been proven to be safe and effective for the PREVENTION of new HPV infections by producing HPV-specific neutralizing antibodies in our circulation and secretions. Once cells are infected by HPV, however, they are detected and eliminated primarily by HPV-specific cytotoxic T lymphocytes via the help of dendritic cells. As such these preventive vaccines cannot be used to TREAT existing HPV infections, which remain highly prevalent in the population for several reasons: the availability of preventive vaccines is relatively recent, many countries have not yet introduced effective national immunization programs, and for those that may have, many people have elected not to be vaccinated. Thus the vaccination uptake rates are highly variable among different countries based on socioeconomic status, geography, culture and other factors. In general, vaccination rates in under-developed, low-income countries are lower than those in developed countries. The vaccination uptake rates in the US and Taiwan are both at about 60% and slowly rising. Japan is a unique example of how difficult it can be to broadly implement HPV vaccination. Since Cervarix© was approved in Japan in October of 2009, HPV vaccination uptake rate among girls quickly rose to >70% in 3~4 years. In 2013 a national immunization program was started based on government recommendation. However, just two months into the program, rare cases of adverse effects reported in the media turned the public opinion against HPV vaccines and led to an indefinite suspension of the government recommendation in June of 2013. Within three years, the vaccination uptake rate in young Japanese girls dropped from 74% to 0.7%. In the meanwhile, the cervical cancer death rate in Japan increased from 3.6% in 1995 to 4.5% in 2018 ref.
Because of these issues with preventive HPV vaccination, national cervical screening programs remain fully in place (with the ongoing introduction of HPV testing) and HPV-related cancer rates continue to rise, as does the need for therapeutic HPV vaccination. If PBI’s therapeutic hrHPV vaccines are shown to be safe and effective treatments for hrHPV infection that prevent the development of precancers, our research shows that patients will be very willing to receive such treatments. Interviews with multiple key opinion leaders (experienced gynecologists) in Taiwan by IQVIA in 2020 showed that if a safe and effective therapy for persistent hrHPV cervical infection is available on the market, their willingness to prescribe it for their patients is about 75% (IQVIA (2020), HPV Therapeutic Vaccine Market Evaluation). It should be emphasized that such early and effective intervention against persistent hrHPV cervical infection carries a tremendous public health impact in reducing healthcare costs related to HPV-driven cancers ref.
Potential opportunities to use vaccines to prevent deaths from cervical cancer