Population-based studies showed that about 60% of individuals will become HPV-infected within five years after first sexual experience, and about 80% of all individuals will become HPV-infected in their lifetime ref. The vast majority of these HPV infections resolve spontaneously within two years, but hrHPV infections have lower rates of spontaneous clearance. When hrHPV infections are not cleared within one year, they are considered as persistent hrHPV infections. Clinical detection of persistent hrHPV cervical infection is achieved through cytologic screening (Pap test) and HPV testing of cervical swabs. The rate of hrHPV-positive cervical swabs tends to be higher in younger than in older women. It also varies between populations based on social factors and testing methodologies, ranging from 5.01% to 18.33% (estimated average at 10%) ref ref ref ref ref. Of all hrHPV cervical infections, HPV16 is the most common (~25%) and this percentage increases significantly in persistent infections as the spontaneous clearance rate of HPV16 is much lower than for most other hrHPV types ref ref. Population-based studies estimate that between 22% and 53% (average 35%) of all cervical HPV infections persist ref ref ref ref.
To estimate the potential US market of persistent hrHPV cervical infections for PBI’s therapeutic vaccines, we start with the estimated number of US women and girls who receive Pap test annually – 22,644,000 ref. Multiply it by the estimated average HPV16-positivity rate of 4.7% ref ref, there were an estimate of 1,064,268 US female individuals with new HPV16 cervical infections, and 35% of these or 372,494 would eventually become persistent infections. Although the US is the leading country in the rollout of PBI’s technology patents, our products’ market is global. In 2017, 17,856,083 Pap tests were done among women in the European Union ref, and the estimated HPV16 prevalence therein is 4.8% ref. Similar calculations give an estimate of 299,982 new persistent HPV16 cervical infections per year in the European Union. These market estimates based on US and European statistics give a total of 672,476 new patients every year who may benefit from PVX2 vaccine therapy for their persistent HPV16 cervical infections. The number of patients who may potentially benefit from PVX2 may be larger due to cross protection and herd effects of vaccination ref, and we are assessing in ongoing studies whether this technology can also treat other hrHPV types. Estimates for HPV16 prevalence by continent are: 2.5% in Asia, 3.5% in Africa, 3.3% in Latin America and Caribbean ref. The worldwide prevalence of HPV16 is 3.3%, and it is the most common hrHPV type detected in all continents ref. As there is no effective medical treatment for persistent hrHPV infections and no competing products are currently on the market, an IQVIA survey conducted in 2020 showed that if there was a safe and effective treatment for persistent hrHPV cervical infection, at least 75% of clinicians would be willing to prescribe it (IQVIA report (2020), HPV Therapeutic Vaccine Market Evaluation).
Potential US market of HPV16 cervical infections (aged 15~59)
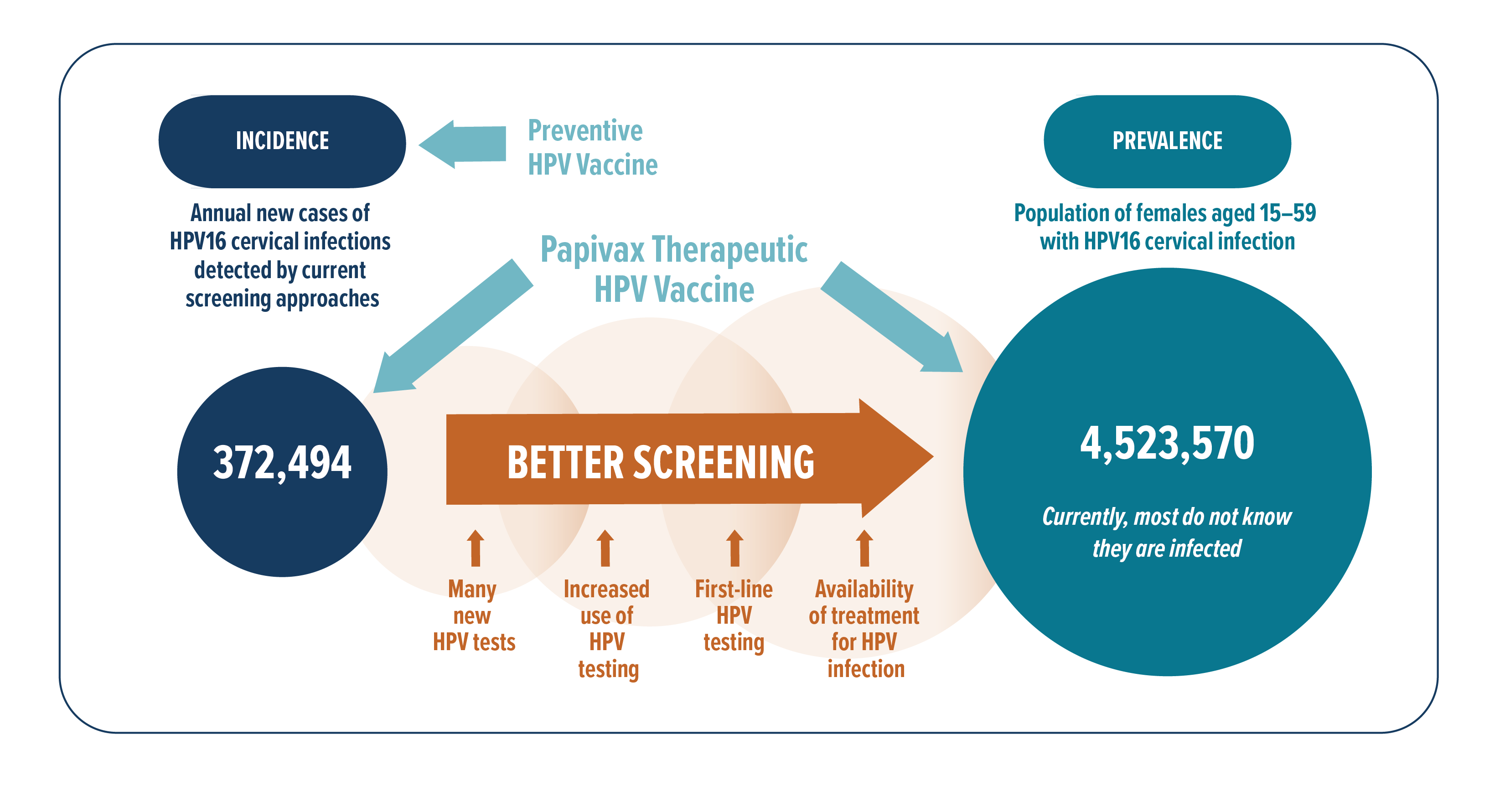
In another epidemiology study ref of US women aged 14~59, HPV16 cervicovaginal infection prevalence rate in 2003~2006 was 4.7%. According to the US Census Bureau, the estimated US female population aged 15~59 in 2019 was 96.25 million. Using these for calculations, we estimate that about 4,523,570 US women aged 15~59 have current HPV16 cervicovaginal infections. Each of these HPV16-infected US girls and women may benefit from receiving a safe and effective immunotherapy, creating a huge potential market for PBI, especially as HPV screening improves in methodology and availability, and as awareness of and demand for treatment by patients and their gynecologists increase.
In addition to the uterine cervix, hrHPV infections of the other anogenital areas and the oral cavity are abundant globally and cause vulvar, anal, vaginal, penile and oropharyngeal cancers. Efforts to develop HPV testing for these patients are advancing rapidly and might soon be administered in routine medical and/or dental office visits, potentially as point-of-care tests. As our recognition of the role hrHPV plays in these prevalent diseases increases, more effective screening methods will arise to allow for their early detection and create more markets for early therapeutic intervention by PVX2 vaccination, including in men.