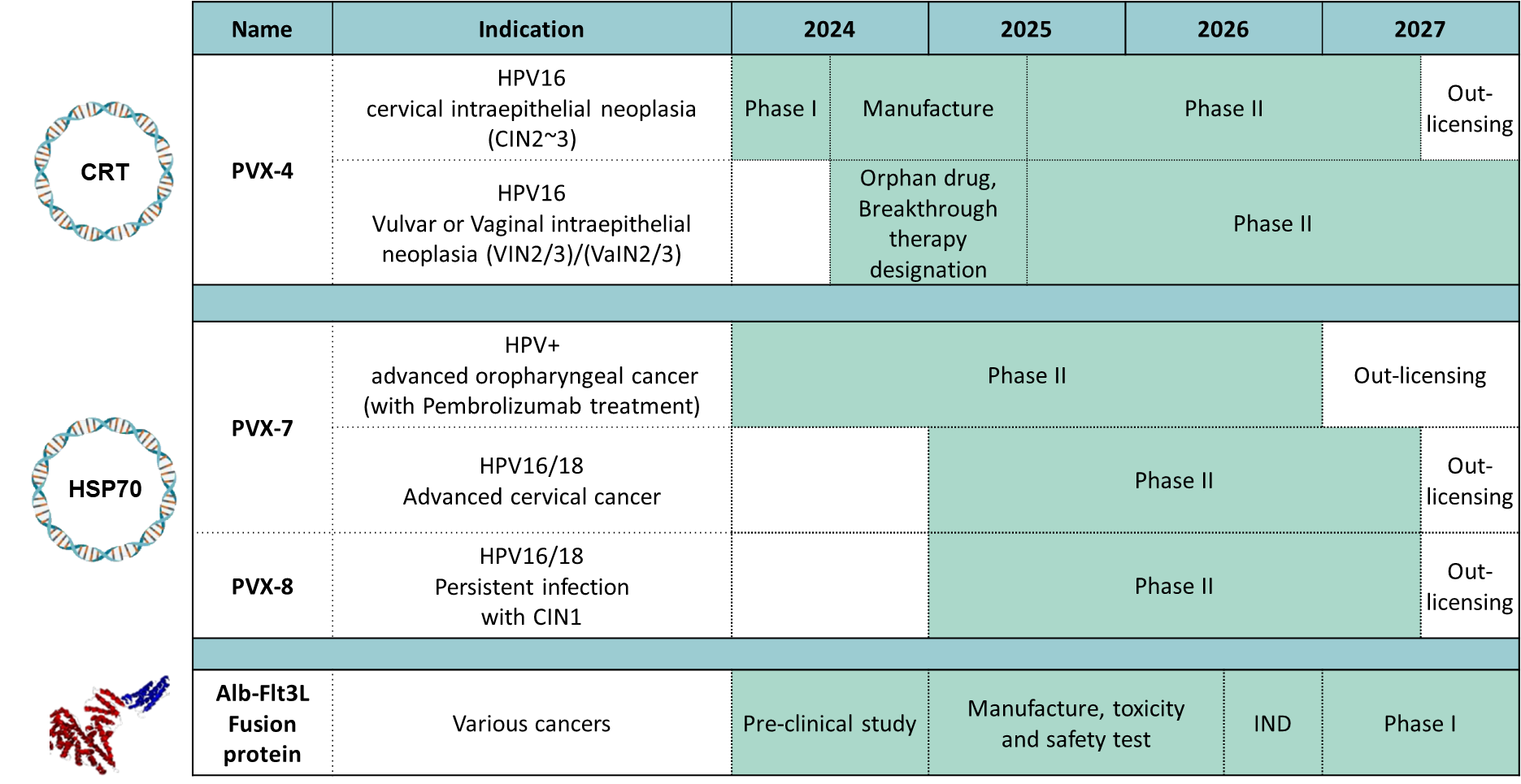
(1) PVX-4
The PVX4 candidate is comprised of a DNA vaccine encoding E6, E7 and L2 genes of the HPV16 virus type fused to the calreticulin (CRT) molecular adjuvant. PVX-4 intended for administration for patients with high grade cervical/vaginal/vluval/anal intraepithelial neoplasia (CIN/VaIN/VIN/AIN 2/3) in order to eliminate the need for the surgery by eliminating HPV16+ cells and prevent new infections. It is administered through intramuscular route with the TriGrid Delivery System. A phase I study is being run at JHU and UAB testing the candidate in women with HPV16+ CIN2/3, and well as a cohort with HIV+ co-infection and CIN2/3, VIN2/3 or VaIN2/3 that is HPV16+. Vaccination has been well tolerated, and there is early evidence of suggesting a remarkably high level of efficacy evidenced by viral clearance and resolution of abnormal pathology in a significant majority of patients (data submitted to SGO Annual Meeting on Women’s Cancer, March 16-18 2024). The PVX-4 Phase I study has been amended to allow enrollment of additional patients in parallel with preparations to initiate late stage clinical testing of the product in early 2025. Based on the extremely encouraging data generated to date, we are exploring a development pathway based on breakthrough therapy, and orphan indications, and have initiated discussions with FDA.
(2) PVX-7
The PVX-7 candidate is comprised of a DNA vaccine encoding E6 and E7 genes of both HPV16 and HPV18 virus types fused to the HSP70 molecular adjuvant (pBI-11 DNA) and a recombinant attenuated vaccinia virus vector (TA-HPV). It is under development for the treatment of human papillomavirus (HPV)-associated cervical cancer and oropharyngeal cancer and is administered through intramuscular route. The regimen includes a heterologous prime-boost immunization regimen, where the pBI-11 DNA vaccine is use as priming agent followed by TA-HPV vaccinia vaccine as booster agent.
PBI has launched an ongoing phase II trial of PVX-7 in patients with recurrent/metastatic oropharyngeal cancer and receiving an immune checkpoint inhibitor (NCT05799144). The candidate has also received US FDA safe-to-proceed determination for a Phase II study women with advanced cervical cancer and receiving an immune checkpoint inhibitor or in the adjuvant setting. PBI is ready to proceed with the adjuvant trial upon securing pending NIH funding.
(3) PVX-8
The PVX-8 candidate is comprised of a DNA vaccine encoding the HPV16 and HPV18 E6 and E7 antigens fused to HSP70 (pBI-11 DNA), which has been manufactured with placebo at cGMP. The pBI-11 DNA can be delivered through needle free device comparing or via the TriGrid Delivery System. We are anticipating the opening of a phase II trial for PVX8 in 2024.
The PVX-8 immunotherapy regimen (pBI-11 DNA vaccine) is to treat patients with known chronic HPV16/18 cervical infection associated with low-grade precancerous lesions (termed ASC-US/ASC-H/LSIL/CIN1 by pathologists). Currently, there is no effective treatment to offer these asymptomatic patients with persistent HPV16/18 cervical infection and early precancerous lesions. Instead they are followed with “watchful waiting” by repeat testing every 6~12 months for high grade precancer or cancer. This causes patients considerable anticipatory anxiety and stigma-related stress and shame ref. Since in only a few of these cases the host immune system will spontaneously clear the HPV infection by 6 months ref, these patients clearly have an unmet need for (and urgently want) a medical treatment for this sexually-transmitted and cancer-causing infection. PBI is treating these patients with PVX-8 to trigger HPV16 and/or HPV18 clearance and thereby eliminate its potential to drive progression to high-grade precancer (CIN2-3) and/or invasive and metastatic cancer.
An overview of the PBI product pipeline is provided below. PBI expects to have Phase II data from all three of it core HPV franchise candidates by Q42026.